The origins of cattle, milk consumption, and lactase persistence
We're going to have to travel quite far back for this one
Greetings Cattle
Cattle, or Bos Taurus to use their latin name, are domesticated bovids, meaning that they are part of the Bovidae family, and share a coevolutionary, mutualistic relationship with humans [1]. Bovids are characterised by having split hooves, a herbivourous diet, and the unique way that they rechew partially degraded food [2].
Neolithisation refers to the process by which humans transitioned from a hunter-gatherer lifestyle to one centered on farming, animal domestication, and permanent settlements. It likely started in an area referred to as the Fertile Crescent, an area spanning the modern-day countries of Iraq, Turkey, Syria, Lebanon, Israel and Palestine [3].
Animal husbandry, the practice of controlled cultivation, management, and production of domestic animals, is thought to have started 15,000 years ago with the domestication of the wolf [4]. Comparing cattle populations between two stages of the Pre-Pottery Neolithic period in the Near East shows that the population decreased, and researchers suggest that this was the effect of domestication rather than other factors, leading us to believe that the first domestication of cattle occurred approximately 10,000 years ago [5].
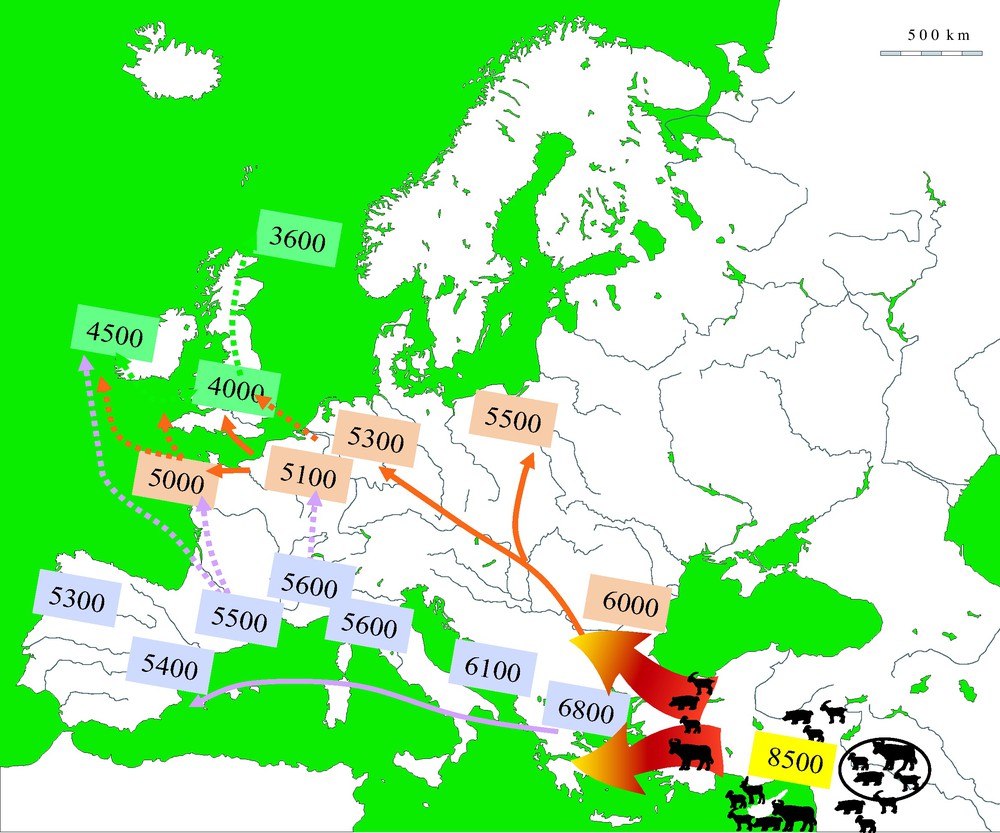
As the Neolithic package was slowly disseminated across Europe, let's take a look at what our ancestors were using cattle for. Firstly, there is strong evidence that suggests their milk was heavily processed into products such as cheese and ghee that were more easily digested by the largely lactose intolerent population at the time, and these milk-derived products had the added benefits of lasting for longer in the event of a surplus [7].
In addition to dairy products, cattle were routinely slaughtered for their meat, which can be inferred by looking at kill-off patterns in archaeological remains [8]. Analysis of a site in Northeastern Bulgaria approximately 8,000 years ago estimated that beef formed approximately 90% of the meat provided by the most frequently identified mammals at the site throughout time [9].
Lactase Non-Persistence vs Lactose Intolerance
Lactose, a compound sugar (disaccharide) composed of galactose and glucose, makes up around 2 - 8% of milk by mass. Mammals are defined by the presence of milk producing mammary glands amongst other key characteristics, and this milk is used to feed their young. Most babies, barring some exceptions [10], produce the enzyme lactase to break down the lactose in milk, but between the natural weaning period, the point at which solid food is introduced into an infant's diet, and adolescence, production of lactase decreases rapidly for two thirds of the world's population. The scientific term for this is lactase non-persistence (LNP).
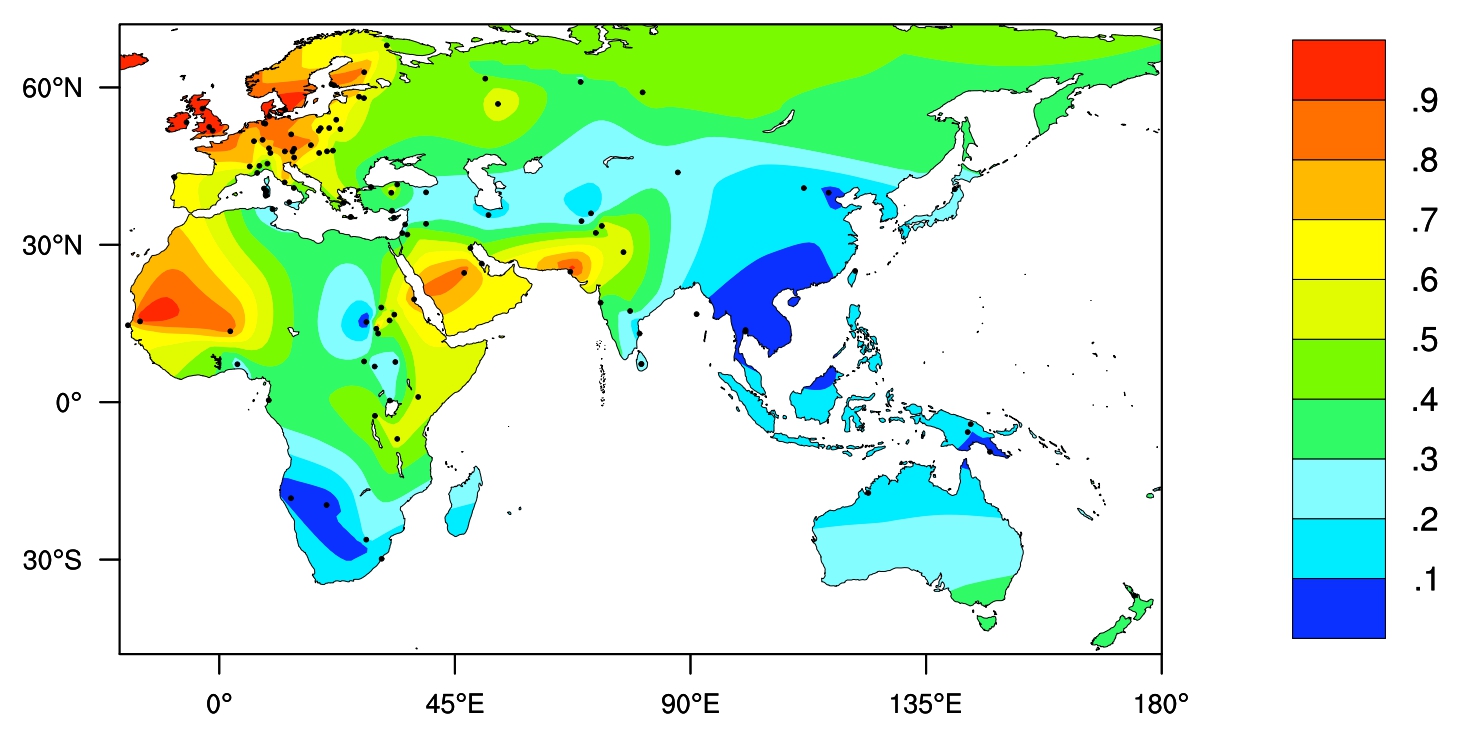
Lactose intolerance on the other hand is a clinical term, and refers to the symptoms that are experienced by an individual who cannot properly digest and absorb lactose. So, are all LNP individuals lactose intolerant? Interestingly not. In China for example, a very high proportion of the population are LNP [12], however milk products are widely consumed, and even recommended by the CDC, The Chinese Center for Disease Control and Prevention. [13]
The Genetic Basis for Lactase Persistence
As we discussed earlier, two thirds of the world's population are lactase non-persistent, which gives rise to the question: What explains the remaining third of the population who do continue to produce lactase after adolescence? It's complicated, and still being researched, but it all relates to a gene mutation that likely first appeared within the last 20,000 years [14] [15].
Let's define a few terms. A phenotype is defined as the set of characteristics of a living thing, resulting from its combination of genes and the effect of its environment, and a genotype is the combination of genes that a particular living thing carries. Humans have around 40,000 different genes [16] and genes are the basic units of heredity, each one controlling a particular quality in living things. The lactase persistence phenotye is simply the observed continued production of the lactase enzyme into adulthood.
Genes are stored on chromosomes, with most humans having 23 pairs, one from each parent, found in the nucleus of nearly every cell in the body. Every chromosome is divided into two arms, a shorter arm (p) and a longer arm (q). On the q arm of the chromosome 2, in a location named 2q21.3, we find the gene LCT, and very close to it (upstream) we find the gene MCM6 [17]. The LCT gene provides the instructions for making the enzyme lactase in cells lining the small intestine [18] and the MCM6 gene is part of the minichromosome maintenance (MCM) complex, involved in the DNA and RNA replication process, but this isn't the relevant part.
Genes contain regions called exons and introns. Exons (expressed regions) make their way into the final product that a gene codes for (a specific protein for example) and introns (intragenic regions) are involved but don't make their way into the final product. The crucial piece of information that explains what MCM6 has to do with the lactase persistence phenotype is the following:

A mutation within intron 13 of the MCM6 gene, designated the −13910 T/C polymorphism (a single-nucleotide change from cytosine to thymine), functions as an enhancer of the LCT promoter - the region responsible for initiating gene expression. This mutation creates a new binding site for Oct-1, a transcription factor (a protein that regulates gene expression). Upon binding, Oct-1 interacts with other transcription factors, forming a complex that activates expression of the LCT gene. This results in the production of lactase. [20].
The −13910 T/C polymorphism was the first gene mutation to be associated with LP, is thought to be the the main driver of LP in Europe, and is one of over 20 currently studied SNPs that underlie the genetic etiology of LP [21].
Interestingly, different populations around the world have individually evolved different genetic mutations that lead to lactase persistence. LP is one of the best examples of convergent evolution, with different genetic mutations producing the same phenotype.





Putting it all together: The Evolution of Lactase Persistence
Now that we have looked at the timeline surrounding animal domestication, dairying practices, and the MCM6 mutations driving lactase persistence in different populations, let's explore some ideas about how the LP phenotype came to be expressed in multiple populations worldwide.
Culture Historical Hypothesis [23]:
The first and most widely accepted theory is the Culture Historical Hypothesis, first suggested in the 1970s, for the following reasons:- There is a significant correlation between lactase persistence (LP) and pastoralism across Old-World populations [14]; pastoralist groups had far greater access to milk than other populations at the time
- There is also high incidence of LNP among adults in the traditional areas of non-milking in East and Southeast Asia, the Pacific and the New World
- There is evidence that suggests that early Neolithic Europeans did not carry the −13910 T/C polymorphism, the mutation widely associated with LP in Northern Europe [24], suggesting that this mutation emerged later
- LP would be a desirable trait as milk could have been used as an alternative food source between periods of crop cultivation, was a relatively pathogen free fluid, and was simply a good source of calories [25]
Calcium Assimilation Hypothesis [26]:
- At high latitudes, incident UVB levels are often too low for adequate vitamin D production, and vitamin D is required for good calcium absorption [27]
- Being calcium deficient puts an individual in danger of developing rickets or osteomalacia
- Milk is a great source of calcium, and also contains vitamin D
- LP was selectively favorable in northern Europe as a means of avoiding poor calcium and vitamin D status
Crisis mechanism [28]:
- Detrimental health consequences of high lactose food consumption by LNP individuals would be acutely manifested during famines
- Lactose-induced diarrhoea can shift from an inconvenient to a fatal condition in severely malnourished individuals
- High lactose (i.e. unfermented) milk products are more likely to be consumed when other food sources have been exhausted
- LP selection pressures would have been greater during times of subsistence instability
Chronic mechanism [28]:
- The establishment of farming communities in Europe resulted in a change in many factors, such as increased population and settlement density, proximity to animals, frequent crop failure, famine, population collapse and general poor hygiene and sanitation
- These factors are likely to have increased infectious disease loads, particularly zoonoses, infections acquired from animals
- Mortality (death) and morbidity (suffering) due to pathogen exposure would have been amplified by the otherwise minor health effects of LNP individuals consuming milk – particularly diarrhoea – due to fluid loss and other gut disturbances, leading to enhanced selection for LP
References
[1]
Zeder, M.A. (2015). Core questions in domestication research. Proceedings of the National Academy of Sciences, 112(11), 3191-3198. View Source
[2]
[3]
University of Chicago News (2023). Fertile Crescent Explained. University of Chicago News. View Source
[4]
Larson, G., Karlsson, E.K., Perri, A. et al. (2012). Rethinking dog domestication by integrating genetics, archeology, and biogeography. Proceedings of the National Academy of Sciences, 109(23), 8878-8883. View Source
[5]
Helmer, D., Gourichon, L., Monchot, H., Peters, J. & Saña, M. (2005). Identifying early domestic cattle from Pre-Pottery Neolithic sites on the Middle Euphrates using sexual dimorphism. Anthropozoologica, 40(1), 19-42. View Source
[6]
Tresset, A. & Vigne, J.D. (2011). Last hunter-gatherers and first farmers of Europe. Comptes Rendus Biologies, 334(3), 182-189. View Source
[7]
Evershed, R.P., Payne, S., Sherratt, A.G., et al. (2008). Earliest Date for Milk Use in the Near East and Southeastern Europe Linked to Cattle Herding. Nature, 455, 528-531. View Source
[8]
Payne, S. (1973). Kill-off Patterns in Sheep and Goats: The Mandibles from Aşvan Kale. Anatolian Studies, 23, 281-303. View Source
[9]
Kamjan, S., de Groene, D., van den Hurk, Y., et al. (2021). The emergence and evolution of Neolithic cattle farming in southeastern Europe: New zooarchaeological and stable isotope data from Džuljunica-Smărdeš, in northeastern Bulgaria (ca. 6200–5500 cal. BCE). Journal of Archaeological Science: Reports, 36, Article 102789. View Source
[10]
Wanes, D., Husein, D. M., & Naim, H. Y. (2019). Congenital Lactase Deficiency: Mutations, Functional and Biochemical Implications, and Future Perspectives. Nutrients, 11(2), 461. View Source
[11]
Itan, Y., Jones, B.L., Ingram, C.J. et al. (2010). A worldwide correlation of lactase persistence phenotype and genotypes. BMC Evolutionary Biology, 10, 36. View Source
[12]
Yongfa, W., Yongshan, Y., Jiujin, X. et al. (1984). Prevalence of primary adult lactose malabsorption in three populations of northern China. Human Genetics, 67, 103–106. View Source
[13]
Chinese Center for Disease Control and Prevention (2022). Nutrition and Health. China CDC. View Source
[14]
Tishkoff, S., Reed, F., Ranciaro, A. et al. (2007). Convergent adaptation of human lactase persistence in Africa and Europe. Nature Genetics, 39, 31–40. View Source
[15]
Bersaglieri, T., Sabeti, P. C., Patterson, N., Vanderploeg, T., Schaffner, S. F., Drake, J. A., Rhodes, M., Reich, D. E., & Hirschhorn, J. N. (2004). Genetic signatures of strong recent positive selection at the lactase gene. American Journal of Human Genetics, 74(6), 1111–1120. View Source
[16]
[17]
UCSC Genome Browser (2023). Human Genome Browser Gateway. University of California Santa Cruz. View Source
[18]
[19]
Jones, B. L., & Swallow, D. M. (2011). The impact of cis-acting polymorphisms on the human phenotype. The HUGO journal, 5(1-4), 13–23. View Source
[20]
Lewinsky, R.H., Jensen, T.G.K., Møller, J., Stensballe, A., Olsen, J., Troelsen, J.T. (2005). T −13910 DNA variant associated with lactase persistence interacts with Oct-1 and stimulates lactase promoter activity in vitro. Human Molecular Genetics, 14(24), 3945–3953. View Source
[21]
Anguita-Ruiz, A., Aguilera, C. M., & Gil, Á. (2020). Genetics of Lactose Intolerance: An Updated Review and Online Interactive World Maps of Phenotype and Genotype Frequencies. Nutrients, 12(9), 2689. View Source
[22]
Ingram, C.I., Montalva, N. and Swallow, D.M. (2022). Lactose Malabsorption. In: Advanced Dairy Chemistry, Volume 3: Lactose, Water, Salts and Minor Constituents, McSweeney et al. (eds.). View Source
[23]
Simoons, F.J. (1970). Primary adult lactose intolerance and the milking habit: A problem in biologic and cultural interrelations. Digestive Diseases and Sciences, 15, 695-710. View Source
[24]
Burger, J., Kirchner, M., Bramanti, B., Haak, W., & Thomas, M.G. (2007). Absence of the lactase-persistence-associated allele in early Neolithic Europeans. Proceedings of the National Academy of Sciences, 104(10), 3736-3741. View Source
[25]
Gerbault, P., Liebert, A., Itan, Y., Powell, A., Currat, M., Burger, J., Swallow, D.M., & Thomas, M.G. (2011). Evolution of lactase persistence: an example of human niche construction. Philosophical Transactions of the Royal Society B: Biological Sciences, 366(1566), 863-877. View Source
[26]
Flatz, G., & Rotthauwe, H.W. (1973). Lactose nutrition and natural selection. Lancet, 302(7820), 76-77. View Source
[27]
DeLuca, H.F. (2004). Overview of general physiologic features and functions of vitamin D. American Journal of Clinical Nutrition, 80(6 Suppl), 1689S-1696S. View Source
[28]
Evershed, R.P., Davey Smith, G., Roffet-Salque, M., Timpson, A., Diekmann, Y., Lyon, M.S., Cramp, L.J.E., Casanova, E., Smyth, J., Whelton, H.L., Dunne, J., Brychova, V., Šoberl, L., Gerbault, P., Gillis, R.E., Heyd, V., Johnson, E., Kendall, I., Manning, K., Marciniak, A., Thomas, M.G. (2022). Dairying, diseases and the evolution of lactase persistence in Europe. Nature, 608(7922), 336-345. View Source